

Article
Notes From ATOPP: Providing Bispecific Antibody Treatments in Community Oncology
July 18, 2024Authors
Topics
The ATOPP Summit covered a range of cutting-edge topics, including the shift toward administering cellular therapies to patients in community oncology settings.
We returned from the Advanced Topics for Oncology Pharmacy Professionals (ATOPP) Summit at the end of June, and the conference covered a range of cutting-edge topics. The panel covering the next generation of cellular therapies caught our attention and got us thinking about exciting advancements in cancer care.
Featuring representatives from Sarah Cannon Cancer Network, University of Chicago, and Florida Cancer Specialists (FCS), the discussion highlighted the rapid approval of new bispecific antibodies (BsAbs), which is pushing integrated delivery networks (IDNs) and community providers to assess the operational challenges associated with these treatments and how they can overcome them to expand access to these new, life-saving treatments. It is essential for manufacturers to understand these operational challenges and provide helpful information to support providers while improving patient access to innovative therapies.
Rapid Advancements in BsAbs
Although one of the first BsAbs, Blincyto (blinatumomab), received US Food and Drug Administration approval a decade ago, recent advancements and approvals are rapidly transforming the treatment landscape, with a substantial number of approvals in the past 2 years (Figure 1). As supply of, and demand for, these treatments increases, IDN providers will need to identify additional clinic and provider capacity to treat patients and may increasingly look to shift select services to lower acuity and community settings.
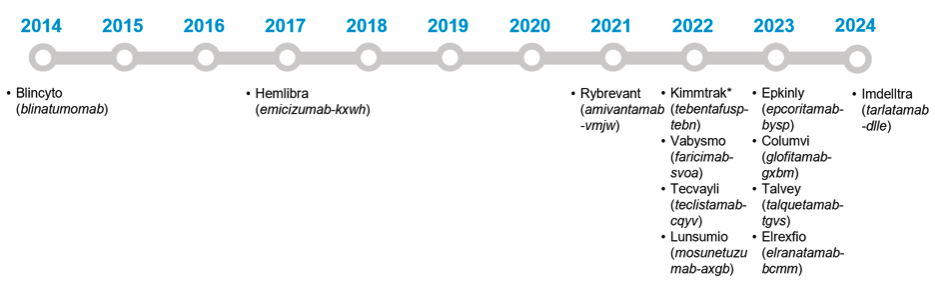
Figure 1. FDA-Approved Bispecific Antibodies.
Abbreviation: FDA, US Food and Drug Administration.
*Note that Kimmtrak is technically a bispecific molecule, not a bispecific antibody.
Source: US Food and Drug Administration. Bispecific antibodies: an area of research and clinical applications. Updated February 2, 2024. Accessed July 18, 2024. https://www.fda.gov/drugs/spotlight-cder-science/bispecific-antibodies-area-research-and-clinical-applications
However, given the adverse effects, operational complexities, and costs of new BsAbs, provider groups are assessing how to expand patient access while managing these challenges and ensuring patient safety.
Increased Demand for BsAb Treatments in Oncology
BsAb treatments have traditionally been delivered by academic health systems and large cancer centers with inpatient beds. Initial doses are generally delivered in the inpatient setting or in the outpatient setting with extensive monitoring. As more BsAbs enter the market, there is increasing demand for institutions that can offer these treatments, and oncologists are eager to increase patient access to these cutting-edge therapies. These forces continue to drive discussion of how to transition these treatments to lower acuity settings or to community oncology practices.
Given clinical risks and operational challenges of delivering BsAb treatments, initial cycles will likely remain at higher acuity centers. However, ATOPP panelists indicated that there are significant opportunities to shift the maintenance phase of care to lower acuity settings, including community practices. For example, the FCS Bispecific Maintenance Therapy program directs patients to receive initial treatment doses at a partner hospital or hospital outpatient site. FCS is then responsible for ongoing maintenance with outpatient monitoring and guidance from FCS physicians.
From our recently published Community Oncology Trend Report, we know that there are already many community oncology practices involved in managing or administering BsAb and chimeric antigen receptor T-cell (CAR-T) therapies. In this year’s survey, we asked community clinics about their involvement in these therapies and found that 85% of the more sophisticated practices surveyed are involved in BsAb or CAR-T treatment, but only 25% stated they fully manage the administration of these therapies independent of a hospital (Figure 2).
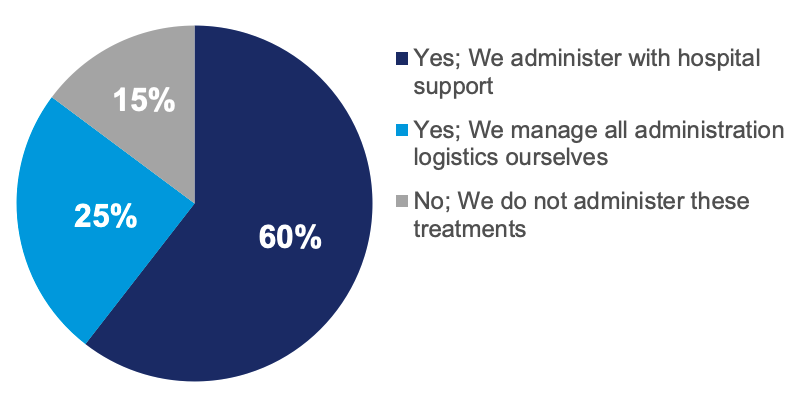
Figure 2. Sophisticated Community Oncology Practices Involvement With Bispecific and CAR-T Therapies.
Chart reflects answers to the question, “Does your organization provide services, observation, or inpatient beds, independently or through a hospital partnership, for patients to whom you administer potentially toxic therapies (bispecifics, CAR-T)?” (N = 102).
Abbreviation: CAR-T, chimeric antigen receptor T cell.
Source: HMP Market Access Insights 2024 Community Oncology Annual Trend Report.
Community practices face several challenges when offering BsAb treatment options, even when focusing only on the maintenance phase:
- Developing protocols: BsAb treatments have important adverse effects, mainly cytokine release syndrome and neurotoxicity, that oncologists and care teams must understand, even during the maintenance phase. Providers and care teams in lower acuity settings may be less familiar with these toxicities. A survey from the Association of Cancer Care Centers found that less than half of oncologists surveyed report having experienced managing neurotoxicity or cytokine release syndrome when administering BsAbs. Community oncology practices must develop protocols for managing these adverse effects and educate care teams.
- Addressing operational challenges: During the maintenance phase, lower acuity care settings and community oncology practices face a multitude of operational challenges when managing patients receiving BsAbs. For example, even during the later cycles of treatment, patients may require monitoring hours that do not fit within standard community practice operating hours. After accounting for treatment delivery and 6 to 8 observation hours, treatment may strain traditional community practice setting hours and operations. Additional operational considerations may include Risk Evaluation and Mitigation Strategy registration and compliance concerns, clinic capacity management, and many more.
- Considering financial impacts: Finally, community oncology practice must consider the financial implications of these programs. These new treatments come with new revenue opportunities but also a variety of challenges to consider, particularly with commercial payers where the specific terms of the letter of agreement must be established. Understanding the opportunity and the risks will be essential to future success.
How Manufacturers Can Support Providers and Expand Access
Manufacturers can play a critical role in supporting providers in lower acuity care settings and community oncology practices with advancing BsAb programs and successfully managing patients during the maintenance phase of treatment. During this period of rapidly increasing demand and the introduction of new programs to meet that demand, manufacturers can support oncologists and care teams by:
- Providing useful and accessible patient educational materials: Given the complexity of these new treatments and the number of new approvals, it is essential that manufacturers provide accurate and engaging patient educational materials. As treatments move to new care settings, providing patient support materials to community practices or lower acuity sites at IDNs will support care teams in directing patients to the right treatments.
- Offering patient management strategies and materials: As discussed above, one of the biggest challenges with expanding more advanced treatments to lower acuity settings is educating oncologists and care teams. Manufacturers can support this process by working with providers to publish patient management strategies or supporting educational events with these aims. To expand access to these advanced treatments, manufacturers may need to target new providers and practices that have not traditionally offered these treatments. Additionally, publishing real-world evidence may also help community practices better understand the adverse events and complication rates patients experience during the maintenance phase.
- Supporting payer engagement: Many of these new treatments are high cost and may face a complex reimbursement landscape. The ATOPP panelists noted spending significant time negotiating coverage and reimbursement with commercial payers. While payers likely have a financial incentive to support transitioning the maintenance phase to lower acuity settings of care, manufacturers can act as partners to providers by offering reimbursement support and ensuring that payers have all required clinical and financial information to support favorable payer policies.
Join us at the Clinical Pathways Congress + Cancer Care Business Exchange in Boston, from September 6-8, 2024, and listen to our panel discussion on what it takes to get bispecifics to market.
If your organization is interested in learning more about our proprietary community oncology study or any of our provider-focused research, please contact Daniel Buchenberger or Emma Bijesse.
The Latest
Podcast
Community Oncology Practice Economics - Podcast Part 2 of 3
In part 2 of this 3-part podcast series, HMP Executive Vice President Lee Blansett and special guest John Hennessy—health system, provider and oncology strategist—explore the operational realities of oncology practice economics.
Lee BlansettPodcast
Community Oncology Practice Economics - Podcast Part 1 of 3
In part 1 of this 3-part podcast series, HMP Executive Vice President Lee Blansett and special guest John Hennessy—health system, provider and oncology strategist—explore the current state of oncology practice economics.
Lee BlansettArticle
Navigating the 340B Maze Amid the Inflation Reduction Act
As the Inflation Reduction Act (IRA) introduces new Maximum Fair Pricing (MFP) rules, integrated delivery networks (IDNs) and manufacturers will face the complexities of navigating overlapping 340B discounts and MFPs.
Emma Bijesse






