

Article
IDN Readout Roundup: Controls, Controls, and More Controls
June 09, 2022Authors
Topics
Welcome to the June 2022 edition of our Monthly Insight Series. This month we highlight several queries that have generated lively discussions thus far.

Greetings from San Mateo!
We enjoyed seeing many of you during our 2022 IDN Oncology Trend Report Readouts last month (and look forward to seeing the rest of you in June!). In addition to following up with many of you to answer your individual questions, we want to highlight several queries that have generated lively discussions thus far.
Should I worry more about order sets or about pathways, in terms of restricting access to my brands?
The short answer is that both order sets and pathways can and will restrict access to branded drugs. HMP Global Market Access Insights (MAI) research shows that the overwhelming majority (almost 90%) of IDNs create and integrate order sets in EHRs to promote clinical consistency and guide treatment selection. Restrictive pathways, defined as pathways limiting oncologists to 1 to 3 treatment options, are less common. Our research indicates only a minority (approximately 1/3rd) of IDNs implement restrictive pathways, and that the most prevalent one, for breast cancer, is used by only 22% of the IDNs surveyed.
Is it true that although restrictive pathways are generally less common, they require higher compliance and cause greater access risk?
Our research indicates a high level of expected compliance in IDNs with pathways. However, many IDNs do not integrate external pathways into the EHR, creating an additional burden on oncologists to view on-pathway options during treatment selection. Furthermore, not all IDNs track pathway compliance, so the ultimate impact on brands may be hard to evaluate. MAI research suggests order sets promote higher levels of compliance from oncologists, given ease of use. Note also that several providers have described pathway guidance as “simple common sense,” making their influence difficult to discern in cases where an oncologist is unconsciously complying with a pathway.
So, what can we do to make sure our brands and product information are getting into order sets, pathways and the EHR?
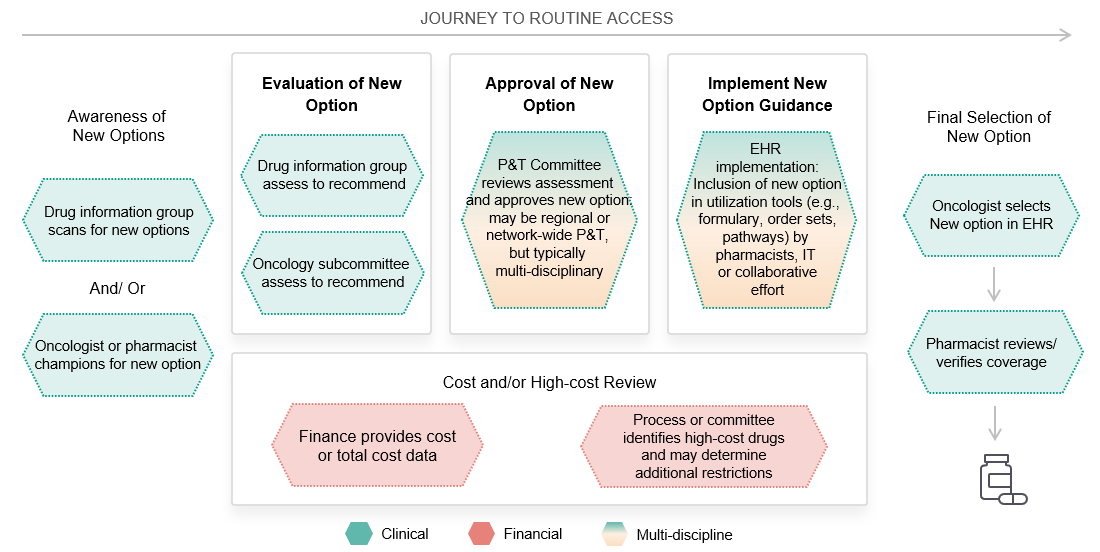
MAI’s research reinforces our concept of a “journey to routine access”: a 3-stage multidisciplinary process. A successful access and engagement strategy requires manufacturers to engage key stakeholders at each stage in a systematic, well-coordinated series of information and support offers.
For more information on the 2022 IDN Oncology Trend Report, stay tuned for your sponsor readout, download our full report on MAI's Client Portal, and take advantage of some the consulting hours included in your subscription
As always, please reach out with any questions or comments you may have.
All the best...
-- HMP Market Access Insights Team
The Latest
Podcast
Community Oncology Practice Economics - Podcast Part 2 of 3
In part 2 of this 3-part podcast series, HMP Executive Vice President Lee Blansett and special guest John Hennessy—health system, provider and oncology strategist—explore the operational realities of oncology practice economics.
Lee BlansettPodcast
Community Oncology Practice Economics - Podcast Part 1 of 3
In part 1 of this 3-part podcast series, HMP Executive Vice President Lee Blansett and special guest John Hennessy—health system, provider and oncology strategist—explore the current state of oncology practice economics.
Lee BlansettArticle
Navigating the 340B Maze Amid the Inflation Reduction Act
As the Inflation Reduction Act (IRA) introduces new Maximum Fair Pricing (MFP) rules, integrated delivery networks (IDNs) and manufacturers will face the complexities of navigating overlapping 340B discounts and MFPs.
Emma Bijesse





